Research Focus
The Hajishengallis laboratory uses diverse and complementary in vitro and in vivo preclinical models and multidisciplinary research approaches to understand the mechanisms that regulate immunity and inflammation in the oral mucosa and how this in turn impacts systemic health and disease. In this manner, the laboratory and its collaborators have made significant novel contributions that challenged earlier paradigms and provided implications and applications above and beyond the oral immunology field.
These include, but are not limited to, the introduction of the keystone pathogen concept; the polymicrobial synergy and dysbiosis model in inflammatory disease; the inflammophilic nature of the dysbiotic oral microbiota; the location-dependent homeostatic principle; the DEL-1–IL-17 balance principle; and the establishment of maladaptive trained myelopoiesis as a mechanistic basis of inflammatory comorbidities.
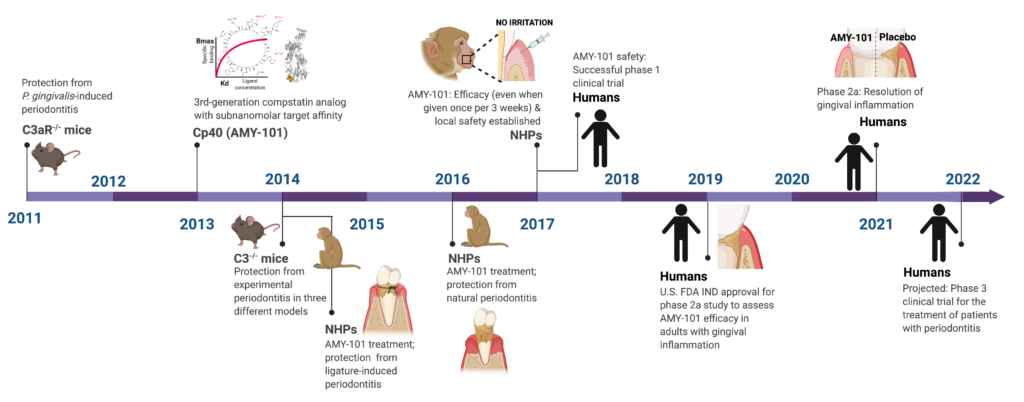
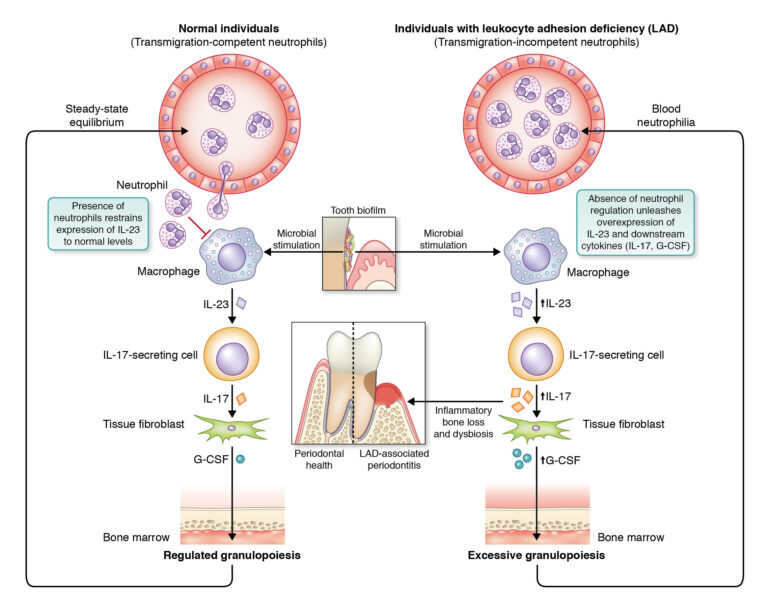
Many of our basic discoveries in model organisms have found application in the treatment of human disease. For instance, our discovery that the central complement component C3 is required for inflammatory periodontal bone loss in different animal models has led to a successful C3-targeted phase 2a clinical trial in patients with periodontal inflammation, with plans for further phase 3 trials (See Figure 1 below). Also of translational importance was our discovery that the aggressive form of periodontitis associated with leukocyte adhesion deficiency type 1 (LAD-1) is not driven by a tissue invasive infection, as traditionally thought, but is rather driven by dysregulation of the IL-23–IL-17 axis (See Figure 2 below). This discovery, made in an animal model of LAD-1, has guided the development of the first successful treatment for this condition in human patients on the basis of antibody-mediated neutralization of IL-23.
Our current research efforts focus on understanding the principles that govern tissue-specific homeostatic immunity, bone regeneration upon inflammation resolution, the impact of aging on progenitor cells and immunity, as well as delving deeper into the mechanisms and consequences of epigenetic inflammatory memory in the context of trained myelopoiesis.